BACTERIOSTATIC SALINE
$6.00
bacteriostatic saline, Provides your body with needed sodium (salt) when you are dehydrated or cannot eat food by mouth. You also may need this….in stock
bacteriostatic saline
Bacteriostatic saline for botox, Bacteriostatic Water (bacteriostatic NaCl) for Injection is a preparation is used only for diluting or dissolving drugs for intravenous, intramuscular, or subcutaneous injection, according to instructions of the manufacturer of the drug to be administered. Bacteriostatic water is available in generic form.
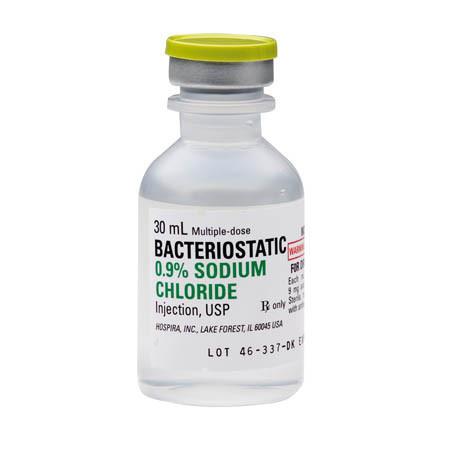
DESCRIPTION
This preparation is designed for parenteral use only after addition of drugs that require dilution or must be dissolved in an aqueous vehicle prior to injection. Bacteriostatic 0.9% Sodium Chloride Injection, USP is a sterile, nonpyrogenic, isotonic solution of sodium chloride in water for injection. Each milliliter (mL) contains sodium chloride 9 mg and 0.9% (9 mg/mL) benzyl alcohol added as a bacteriostatic preservative. May contain hydrochloric acid for pH adjustment. It is supplied in a multiple-dose container from which repeated withdrawals may be made to dilute or dissolve drugs for medication. The pH is 5.0 (4.5 to 7.0).
The semi-rigid vial is fabricated from a specially formulated polyolefin. It is a copolymer of ethylene and propylene. The safety of the plastic has been confirmed by tests in animals according to USP biological standards for plastic containers. The container requires no vapor barrier to maintain the proper drug concentration.
USES:
- Provides your body with needed sodium (salt) when you are dehydrated or cannot eat food by mouth. You also may need this medicine if you have certain metabolic or nutrition problems, or serious medical problems such as AIDS, cancer, or burns.
SIDE EFFECTS
Reactions which may occur because of this solution, added drugs or the technique of reconstitution or administration include febrile response, local tenderness, abscess, tissue necrosis or infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection and extravasation.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate counter-measures, and if possible, retrieve and save the remainder of the unused vehicle for examination.
Although adverse reactions to intravenous, intramuscular or subcutaneous injection of 0.9% benzyl alcohol are not known to occur in man, experimental studies of small volume parenteral preparations containing 0.9% benzyl alcohol in several species of animals have indicated that an estimated intravenous dose up to 30 mL may be safely given to an adult without toxic effects. Administration of an estimated 9 mL to a 6 kg infant is potentially capable of producing blood pressure changes.
DOSAGE AND ADMINISTRATION
The volume of the preparation to be used for diluting or dissolving any drug for injection is dependent on the vehicle concentration, dose and route of administration as recommended by the manufacturer. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. See PRECAUTIONS.
| Class | Rx |
| Medical License Required | Yes |
| Container | Fliptop Vial – Plastic |
| Form | SDV |
| Generic Name | Sodium Chloride |
| Preservative | No |
| Size | 10 mL |
| Strength | 0.009 |
| Therapeutic Class | Replacement Solution |
| Unit of Measure | Vial |
Be the first to review “BACTERIOSTATIC SALINE” Cancel reply
Related products
Botulinums
Botulinums
Botulinums
Botulinums
Botulinums
Botulinums
Botulinums
Botulinums













Reviews
There are no reviews yet.